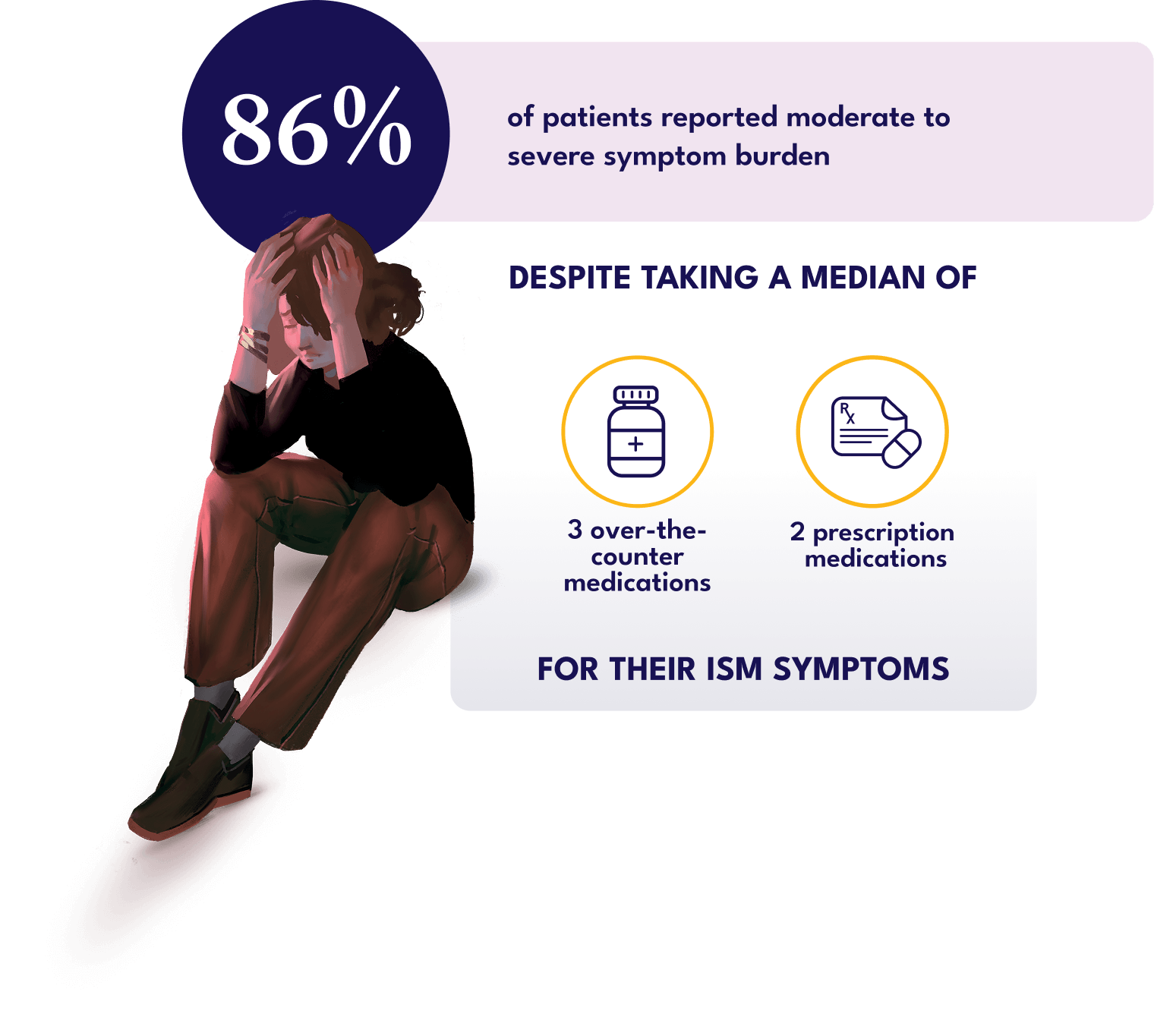
What you may not see is that the symptom burden of systemic mastocytosis (SM), including the indolent subtype, can significantly disrupt patients’ lives1,2
Indolent systemic mastocytosis (ISM) represents ~90% of SM cases,3 and the symptoms of ISM can impact patients’ work, social lives, and overall well-being1,2
“I tend to isolate when I have more symptoms. I stay in a lot, and it can feel depressing.”
Teri, a patient describing living with ISM
Watch the video to learn from Kristine and Sarah, 2 patients living with SM, about how ISM impacts their everyday lives

- These individuals receive compensation from Blueprint Medicines for sharing their experience living with systemic mastocytosis through the Blueprint Medicines SM Ambassador Program.
“I’ve taken many different
medications, and found
that my symptoms just
weren’t resolved.”
Teri, a patient describing living with ISM
The Mastocytosis Control Test (MCT) helps assess
disease control - a tool that may be useful to support
conversations with your patients.6†‡

Could ongoing uncontrolled disease signal a need to reassess your patients' disease management strategy?
EXPLORE A TREATMENTOPTION FOR ISM